By Steven Lumley, technical manager, WearCheck
Metal deactivators play a key role in the fight against corrosion. This is the next focus in the ongoing discussion about lubricant additives, with condition monitoring specialist company, WearCheck.
| What are metal deactivators? | Organic complexes containing nitrogen or sulphur, amines, sulphides and phosphites
|
| What do they do? | Reduce catalytic effect of metals on oxidation rate
|
| How do they do it? | Form an inactive film on metal surfaces by complexing with metallic ions
|
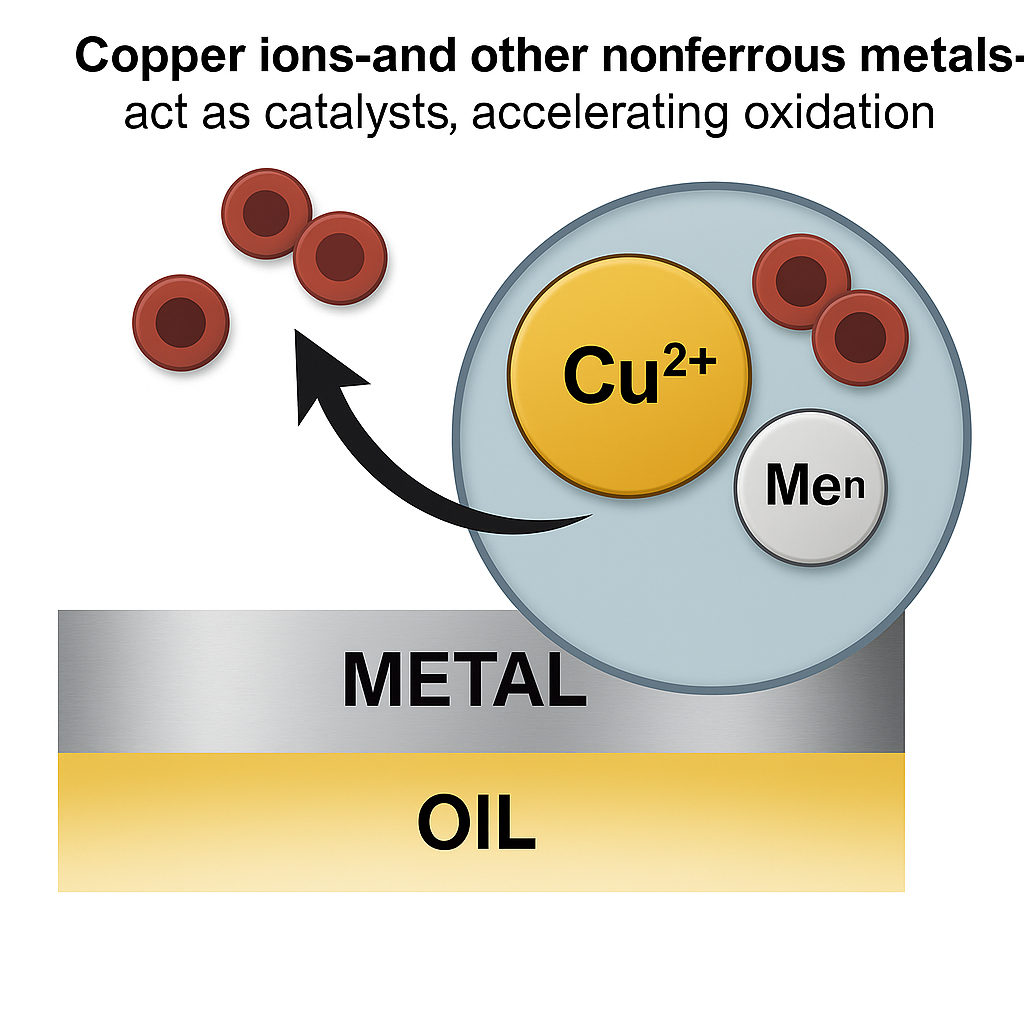
Metal deactivators are another important additive in lubricating-oil formulations, especially with the growing use of non-ferrous metals, notably copper- and aluminium-containing alloys, that can be prone to the negative effects of staining and corrosion.
These metals (or more specifically their ions) are also catalytically active, and act as reaction sites that can trigger lubricant degradation through oxidation of the base oil.
Metal deactivators belong to the corrosion-inhibitor class of additives, and they work their magic on metal surfaces, especially non-ferrous metals, but also do their thing with wear particles that are dissolved or suspended in the oil.
These specialised additives contain surface-active molecules that adsorb on metal surfaces. The best-understood mechanism for how they function involves the formation of an inactive barrier film that inhibits cathodic and/or anodic reactions that can accelerate oxidation and cause corrosion.
Metal deactivators are typically organic compounds that contain nitrogen or sulphur atoms. These atoms have a high affinity for metal ions and can form strong, stable bonds with them. This allows the additives to neutralise or deactivate these metal ions in the oil, which reduces their negative effects.
Lubricant blenders add metal deactivators to oils, primarily to protect copper and yellow metals in most automotive and industrial lubricants, including greases and metal-working fluids.
But wait – there’s more! The protective film that metal deactivators form on a metal surface is also thermally stable, water insoluble and chemically bonded.
Please visit www.wearcheck.co.za or email marketing@wearcheck.co.za for more information.







